eBlocks™ Gene Fragments
Assemble multiple constructs for screening with dsDNA sequences delivered quickly and within budget. These high-quality gene fragments are designed for fast turnaround times and can ship in as little as 1–3 business days from order confirmation.
Ordering
- Fast turnaround
- Minimal screening effort
- Budget friendly
eBlocks Gene Fragments
Shipped in 1-3 business days of order confirmation (excluding Fridays).
Orders require a minimum of 24 fragments per plate*.
Table 1. Available formats of eBlocks Gene Fragments.
| Plate type | Buffer option | Volume | Concentration |
|---|---|---|---|
| 96-well PCR Plate | Nuclease-free water or IDTE pH 8.0 | 20 µL | 10 ng/µL |
| Dry (no buffer) | N/A | N/A | |
| 384-well PCR Plate | Nuclease-free water or IDTE pH 8.0 | 20 µL | 10 ng/µL |
| 384-well Echo™ Plate** | Nuclease-free water or IDTE pH 8.0 | 50 µL | 4 ng/µL |
To achieve the fastest possible shipping time, items that do not meet IDT's purity and yield specifications after the first assembly attempt will be cancelled at no charge. No formal cancellation notice will be sent for these items. However, cancellations will be noted on the item specification sheet, which can be accessed using the Synthetic Biology Order Status Checker.
*Plates with less than 24 fragments will incur an additional fee applied at checkout
**Echo is a trademark of Labcyte Inc. A Beckman Coulter company
Product details
eBlocks Gene Fragments are double-stranded DNA fragments of 300–1500 bp in length that are typically shipped in 1–3 business days. They are uniquely suited for high-throughput screening of multiple constructs.
IDT offers fragments without flanking (i.e., universal adapter) sequences at no extra cost. With no flanking sequences to remove, eBlocks are ready-to-use for various applications.
Table 2. eBlocks Gene Fragment characteristics.
| Specification | Information |
|---|---|
| Length | 300–1500 bp |
| Median error rate | 1:5000 bp |
| Shipping time* | 1–3 business days |
| Yield | 200 ng |
| Format | Plate |
* Shipping time refers to the number of business days after order confirmation (excluding Fridays for wet plates). Shipping time is dependent on length and complexity of the eBlocks Gene Fragments ordered. In a few cases, shipping time may exceed the estimated time.
Storage conditions
| Short term (<2 weeks) | 4°C |
| Long term (up to 2 years) | –20°C |
Quality control for higher cloning efficiency
Each eBlocks Gene Fragment goes through a quality control process by verifying the size of the fragment by capillary electrophoresis, confirming that most recombinant colonies obtained from cloning contain the desired insert. More complex sequences may require the end-user to sequence additional clones.
Request a consultation
Working on antibody discovery or other high throughput applications you’d like to discuss? Your time is valuable and we’ll prioritize your inquiry. Click on “Request a consultation” to provide brief information about your project, and we’ll be in touch to discuss it ASAP.
Request a consultationProduct data
Minimal screening effort
eBlocks Gene Fragments are compatible with many convenient cloning and assembly kits. eBlocks Gene Fragments reduce the time needed and expense of screening colonies.
The values in Table 3 demonstrate the typical screening requirements when using an isothermal assembly method under optimal conditions.
Table 3. Low screening effort is needed for eBlocks Gene Fragments to have a >90% chance to find the correct clone.
| Length (bp) | Colonies to pick |
|---|---|
| 300–499 | 2 |
| 500–1500 | 3 |
Consistent high fidelity
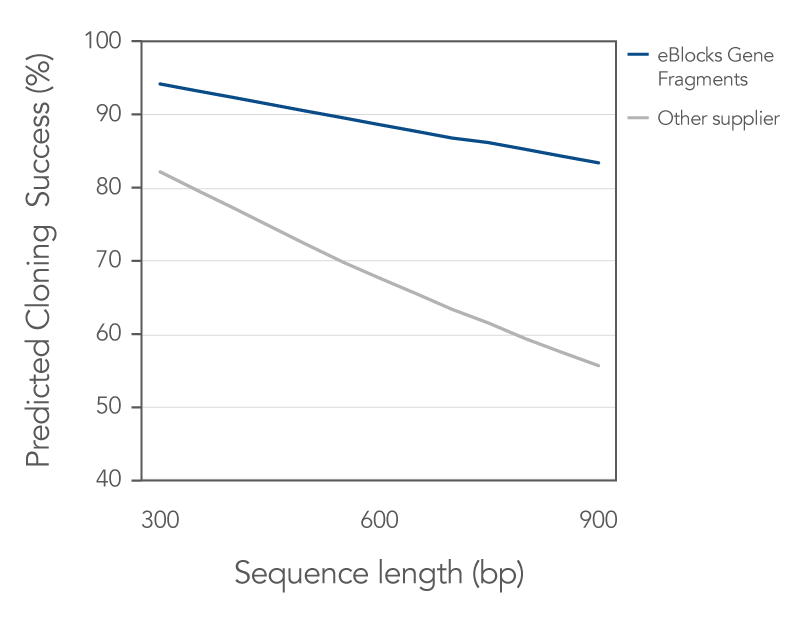
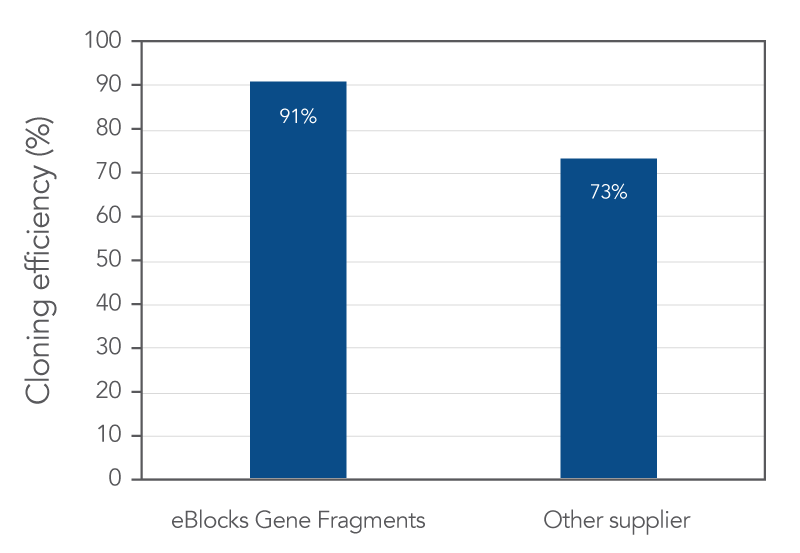
All IDT gene fragments demonstrate a consistent, high sequence fidelity across various lengths, and eBlocks Gene Fragments are no exception (Figures 1 and 2).
Figure 1. In this study, eBlocks Gene Fragments had a higher predicted cloning success compared to fragments supplied by an alternative vendor. eBlocks Gene Fragments have a median error rate of less than 1:5000 bp. This means that eBlocks Gene Fragments demonstrate a high probability of first-time cloning success, indicating that IDT fragments may be more likely to give a correct clone the first time than fragments supplied by the other vendor at comparable length ranges.
Across various lengths, IDT gene fragments demonstrated consistent cloning efficiency and cloning fidelity. In a recent head-to-head comparison study, IDT fragments out performed those from another supplier. Read the study to learn more about how DNA synthesis and sequence error correction processes at IDT positively impact fidelity and screening efforts compared to alternative supplier.
Resources
Frequently asked questions
What should I do if the IDT online ordering tool says that my sequence is too complex to order, but I need it as is?
In most cases, our system has found a region with either very high G/C content or several small repeats that would prevent us from synthesizing your sequence.
If your sequence contains a coding region, you can use codon optimization to reduce the complexity. Codon optimization uses synonymous codons to retain the final amino acid sequence that is expressed while generating a sequence that we can synthesize.
If your sequence is not a coding region, we might be able to synthesize the sequence as a custom gene, which is delivered in a basic pUC vector, rather than just a linear, dsDNA fragment. For assistance, send your sequences and any questions to:
amr-applicationsupport@idtdna.com (for customers in the United States or Canada)
emea-applicationsupport@idtdna.com (for customers in Europe)
apac-applicationsupport@idtdna.com (for customers in Asia Pacific, Australia, New Zealand, Japan, and South Korea)
Can I order eBlocks™ Gene Fragments in a plate format other than what is listed?
We can often support shipping in alternate plate types. In some cases, this may increase cost and turnaround time.
To see if your plate type is an option for eBlocks synthesis, email:
amr-applicationsupport@idtdna.com (for customers in the United States or Canada)
emea-applicationsupport@idtdna.com (for customers in Europe)
apac-applicationsupport@idtdna.com (for customers in Asia Pacific, Australia, New Zealand, Japan, and South Korea)
Do you recommend using eBlocks™ Gene Fragments to assemble larger constructs?
eBlocks Gene Fragments are designed to be used for high-throughput screening methods.
We do not recommend attempting to assemble multiple eBlocks fragments to make a larger construct.
Should I amplify my eBlocks™ Gene Fragments when I receive them?
We do not recommend amplifying after receipt. eBlocks Gene Fragments are synthesized from several smaller overlapping fragments. Even after purification, the final prep may contain some of these smaller byproducts.
During PCR smaller products are more efficiently amplified than longer ones, and once the exponential phase of PCR is reached, any smaller products in the reaction will overwhelm the generation of the full-length correct product. Researchers will often see a smear or smaller bands than expected when the PCR product is run on a gel.
What is the difference between a gBlocks™ Gene Fragment and an eBlocks™ Gene Fragment?
Here is a comparison of gBlocks and eBlocks Gene Fragments:
| gBlocks Gene Fragments | eBlocks Gene Fragments | |
|---|---|---|
| Double-stranded DNA | ✓ | ✓ |
| Stringent QC process • Median error rate • Synthesis & assembly |
1:5000 May rework to meet QC standards |
1:5000 No rework† |
| Format | Plates or tubes | Plate |
| Length | 125–3000 bp | 300–1500 bp |
† Any eBlocks Gene Fragment that does not meet QC standards after the first attempt will be cancelled and refunded.
Can I order eBlocks™ Gene Fragments in a tube format?
Unfortunately, we only support shipping eBlocks Gene Fragments in plate format at this time.
How do I design my eBlocks™ Gene Fragment for restriction cloning?
Cloning of eBlocks Gene Fragments is similar to cloning a PCR product. For restriction cloning, it is important to add 6–8 nt at the ends of the fragment, beyond the restriction recognition sequence.
Most restriction enzymes will not cut efficiently, or at all, if the recognition site is directly on the fragment's terminal end.
Do you provide a codon optimization service?
IDT has a free, online codon optimization tool available through our SciTools™ Web Tools suite that you can use to perform codon optimization before ordering your oligonucleotide.
To optimize, simply paste in an amino acid or nucleotide sequence and select your desired expression host. If you need additional assistance, contact us.
Biosecurity
Sequence Information is secure and confidential at IDT. Please see our Confidentiality Statement for more information. All online ordering steps, including sequence entry and your choice of parameters, are also secure and protected.
We screen the sequence of every gene, and gene fragment order we receive to (1) identify any regulated and other potentially dangerous pathogen sequences, and (2) verify that IDT’s gene customers are legitimate scientists engaged in beneficial research.
IDT is among the five founding members of the International Gene Synthesis Consortium (IGSC) and helped to create the IGSC’s Harmonized Screening Protocol. The Harmonized Screening Protocol describes the gene sequence and customer screening practices that IGSC member companies employ to prevent the misuse of synthetic genes. IDT takes the steps set out in the Harmonized Screening Protocol to screen the sequences of ordered genes and the prospective customers who submit those orders.
For more information about the IGSC and the Harmonized Screening Protocol, please visit the website at www.genesynthesisconsortium.org.

In October 2010, the United States government issued final Screening Framework Guidance for Providers of Synthetic Double-Stranded DNA, describing how commercial providers of synthetic genes should perform gene sequence and customer screening. IDT and the other IGSC member companies supported the adoption of the Screening Framework Guidance, and IDT follows that Guidance in its application of the Harmonized Screening Protocol. For more information, please see 75 FR 62820 (Oct. 13, 2010), or https://federalregister.gov/a/2010-25728.